Hearing aid batteries utilize a zinc/air electrochemical cell. The reduction half-reactions and standard reduction potentials for this cell are given below. What species are oxidized and reduced in the cell reaction? 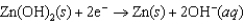 -1.249 V
-1.249 V 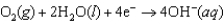 +0.401 V
+0.401 V
A) Oxygen is reduced and zinc is oxidized.
B) Zinc is reduced and oxygen is oxidized.
C) Zinc hydroxide is reduced and oxygen is oxidized.
D) Zinc hydroxide is reduced and hydroxide is oxidized.
E) Water is reduced and zinc is oxidized.
Correct Answer:
Verified
Q61: Barium is a nonessential element in
Q62: Pacemakers implanted in the chests of
Q63: The cation BiO+ is found in some
Q64: Rhenium-186 has medical applications. It decays
Q65: Thallium-201 is used in cardiac imaging. It
Q67: Radioactive tritium, 3H, is used as
Q68: Which element may contribute to sudden infant
Q69: Lithium is used in the treatment of
Q70: Pacemakers implanted in the chests of
Q79: A patient is injected with a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents