Hearing aid batteries utilize a zinc/air electrochemical cell. The reduction half-reactions and standard reduction potentials for this cell are given below. Write the balanced reaction equation for the cell and report the sum of the stoichiometric coefficients. 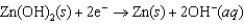 -1.249 V
-1.249 V 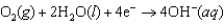 +0.401 V
+0.401 V
A) 4
B) 5
C) 6
D) 7
E) 8
Correct Answer:
Verified
Q69: Lithium is used in the treatment of
Q70: Pacemakers implanted in the chests of
Q72: The release of radioactive cesium-137 into the
Q73: The isotope 18F is used in
Q75: The isotope 201Th is used in
Q76: A radioactive isotope that emits the shortest
Q77: The essential elements found in the human
Q78: Hearing aid batteries utilize a zinc/air
Q79: A patient is injected with a
Q79: A patient is injected with a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents