Hydroxyapatite, which is a component of tooth enamel, is very insoluble in water. For the following reaction, Ksp = 2.3 10-59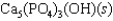
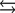
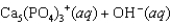 What is the molar concentration of Ca5(PO4)3+ when the pH of the mouth is 4.0?
What is the molar concentration of Ca5(PO4)3+ when the pH of the mouth is 4.0?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q82: Why are the radioactive isotopes of cesium
Q83: Hearing aid batteries utilize a zinc/air
Q84: Nitrogen is a major essential element in
Q85: Different concentrations of ions on either
Q86: Why are some elements in the human
Q88: Thallium-201 is used in cardiac imaging. It
Q89: Fluoride is added to toothpastes and drinking
Q90: Ions need to move in and out
Q91: Different concentrations of ions on either
Q92: The photosynthesis of carbohydrates from carbon dioxide
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents