The following reaction occurs in basic solution. Identify the reducing agent. Note that the reaction equation is not balanced. 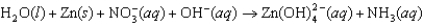
A) Zn(s)
B) NO3-(aq)
C) OH-(aq)
D) H2O(l )
E) Zn(OH) 42-(aq)
Correct Answer:
Verified
Q6: Oxidation is the _
A)gain of electrons.
B)loss of
Q7: Where in the periodic table do you
Q8: When aluminum metal is obtained from aluminum
Q9: Reduction refers to _
A)a decrease in oxidation
Q10: What is the oxidation number of chromium
Q12: The following reaction occurs in basic solution.
Q13: The following reaction occurs in a new
Q14: Which one of the following items does
Q15: Glancing at a periodic table, where do
Q16: Which one of the following items does
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents