A voltaic cell is constructed based on the oxidation of zinc metal and the reduction of silver cations. Solutions of silver nitrate and zinc nitrate also were used. Locate the salt bridge in the diagram. 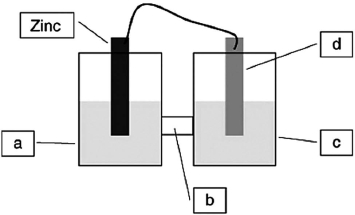
A) a
B) b
C) c
D) d
Correct Answer:
Verified
Q19: The following reaction occurs in a new
Q20: Reduction is the _
A)gain of electrons.
B)loss of
Q21: A voltaic cell is constructed based on
Q22: Which statement about a cathode in a
Q23: Proteins containing a certain functional group (identified
Q25: The diagram below represents a voltaic cell.
Q26: Consider the following chemical reaction used to
Q27: Which statement about a voltaic cell is
Q28: The electrodes on batteries are labeled +
Q29: The diagram below represents a voltaic cell.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents