An electrochemical cell is constructed with a zinc metal anode in contact with a 0.052 M solution of zinc(II) nitrate and a silver cathode in contact with a 0.0042 M solution of silver(I) nitrate. What is the emf of this cell at 5 C? 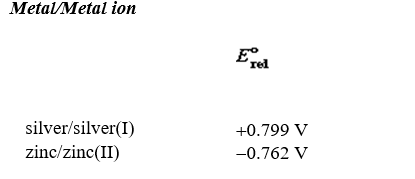
A) 1.656 V
B) 1.465 V
C) 1.561 V
D) 1.370 V
E) 1.609 V
Correct Answer:
Verified
Q80: If the potential of a voltaic cell
Q81: An electrochemical cell is represented below. Which
Q82: The standard cell potential for the nickel-cadmium
Q83: What is true when a battery (voltaic
Q84: Cadmium is a toxic heavy metal.
Q86: An electrochemical cell at 298 K is
Q87: Lead is a toxic metal. The
Q88: Which statement about this concentration cell is
Q89: An electrochemical cell has both a silver
Q90: Electrochemical cell potentials can be used
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents