Cadmium is a toxic heavy metal. The U.S. Environmental Protection Agency limits the concentration of cadmium ions in drinking water to less than 4 10-8 M. Assess the feasibility of monitoring of cadmium in drinking water using a voltaic cell based on the following half-reactions. 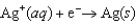 E = +0.7996 V
E = +0.7996 V 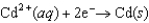 E = -0.4030 V
E = -0.4030 V
If the silver ion concentration were 0.100 M, what cadmium ion concentration at 298 K would increase the cell potential by 10 mV from the standard cell potential?
A) 3.6 10-2 M
B) 4.6 10-2 M
C) 4.6 10-3 M
D) 6.8 10-3 M
E) 6.8 10-2 M
Correct Answer:
Verified
Q93: What is the cell potential for this
Q94: A pH meter uses an electrode arrangement
Q95: Zinc-air batteries are actively being researched
Q96: Neuron cells generate electrical signals by concentration
Q97: Electrochemical cell potentials can be used
Q99: A concentration cell is constructed by using
Q100: An electrochemical cell is constructed with
Q101: The unit of electrical power, watt (W),
Q102: The average electrical current delivered if
Q103: Calculate the value of K for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents