Lead is a toxic metal. The U.S. Environmental Protection Agency limits the concentration of lead ions in drinking water to less than 5 10-8 M. Assess the feasibility of monitoring of lead in drinking water using a voltaic cell based on the following half-reactions. 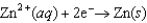 E = -0.762 V
E = -0.762 V 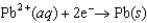 E = -0.126 V
E = -0.126 V
If the zinc ion concentration were 0.100 M, what lead ion concentration at 298 K would decrease the cell potential by 10 mV from the standard cell potential?
A) 0.012 M
B) 0.22 M
C) 0.046 M
D) 0.15 M
E) 0.022 M
Correct Answer:
Verified
Q82: The standard cell potential for the nickel-cadmium
Q83: What is true when a battery (voltaic
Q84: Cadmium is a toxic heavy metal.
Q85: An electrochemical cell is constructed with
Q86: An electrochemical cell at 298 K is
Q88: Which statement about this concentration cell is
Q89: An electrochemical cell has both a silver
Q90: Electrochemical cell potentials can be used
Q91: For the following reaction, predict the
Q92: When a voltaic cell reaches equilibrium, _
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents