If the free-energy change of the following voltaic cell 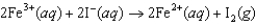
is -46.3 kJ, what is the standard potential of the cell?
Correct Answer:
Verified
Q153: From the following table of standard reduction
Q154: What is the cell potential for a
Q155: Electrochemical cell potentials can be used to
Q156: Calculate the cell potential (Ecell) for the
Q157: Silver tarnishes due to the formation of
Q159: How much work can be done using
Q160: For the electrochemical cell indicated below, in
Q161: How does a fuel cell differ from
Q162: Chromium often is electroplated on other metals
Q163: The capacity of a battery usually
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents