Permanganate ion can oxidize sulfite in basic solution according to the following equation. The relevant standard reduction potentials are 0.59 V for the manganese compound and -0.92 V for the sulfur compound. Determine the cell potential for the reaction at 298 K with the concentrations in the table. 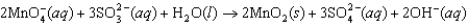
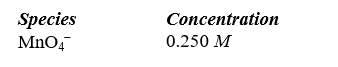

Correct Answer:
Verified
Q145: For the following electrochemical cell, write the
Q146: Using the following data, determine the standard
Q147: What is the change in free energy
Q148: What constitutes a standard hydrogen electrode?
Q149: For the following electrochemical cell, draw and
Q151: What is the most important use for
Q152: The following reaction is called the "super
Q153: From the following table of standard reduction
Q154: What is the cell potential for a
Q155: Electrochemical cell potentials can be used to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents