For the following reaction, predict the pH if the pressure of O2 is 3.00 atm and the bromide ion concentration is 2.10 M. The measured cell potential (Ecell) was 0.100V. 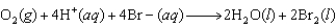
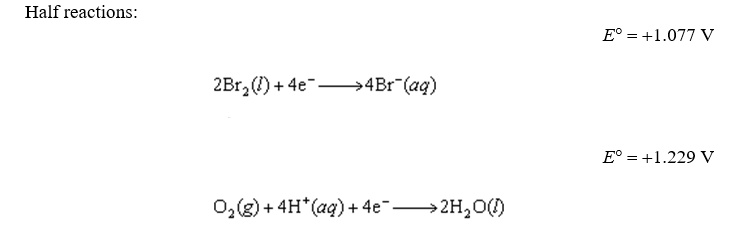
Correct Answer:
Verified
Q139: Which of the following is not an
Q140: Which statement regarding battery-powered electric cars is
Q141: What kind of chemical reaction occurs at
Q142: Starting with
Q143: What must be true about the standard
Q145: For the following electrochemical cell, write the
Q146: Using the following data, determine the standard
Q147: What is the change in free energy
Q148: What constitutes a standard hydrogen electrode?
Q149: For the following electrochemical cell, draw and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents