When a molecule of ethylenediamine replaces two molecules of NH3 in Co(NH3) 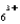 , the entropy of the system ________
, the entropy of the system ________
A) increases.
B) decreases.
C) remains the same.
D) cannot be determined.
E) is irrelevant.
Correct Answer:
Verified
Q16: Which, if any, of statements A-D is
Q17: In a spontaneous process, the entropy of
Q18: The entropy change in the surroundings
Q19: An ice cube at 0
Q20: The enthalpy of fusion for benzene
Q22: Consider a closed container containing a 1
Q23: Indicate which one of the following
Q24: Perfect crystals of carbon monoxide (CO) are
Q25: Before class, students were distributed throughout a
Q26: Indicate which one of the following reactions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents