In an experiment, 1.00 atm of N2(g) in a 10.0 L container at 25 C was reacted under standard state conditions with a stoichiometric quantity of H2(g) to form ammonia: 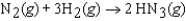 .
.
What is the entropy change for the reaction? 
A) (-198.7 J/K)
B) (-81.5 J/K)
C) (-27.2 J/K)
D) (+81.5 J/K)
E) (+198.7 J/K)
Correct Answer:
Verified
Q37: The gas above the liquid in
Q38: The following figures represent distributions of gas
Q39: Which of the following must be
Q40: Indicate which of the following has
Q41: The standard molar entropy of magnesium fluoride
Q43: NO gas is converted to NO2 gas
Q44: The entropy of a NaCl crystal is
Q45: Indicate which of the following has the
Q46: Indicate which of the following has
Q47: The symbol
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents