Multiple Choice
Determine the value of G for the reaction at 298 K. 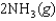
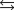
 Given
Given 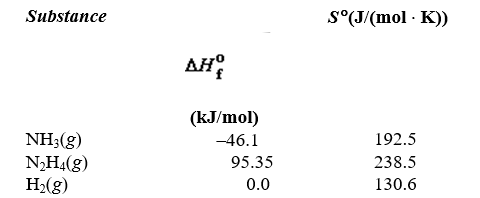
A) (-88.8 kJ)
B) (+88.8 kJ)
C) (+192 kJ)
D) (-192 kJ)
E) (-3.38 kJ)
Correct Answer:
Verified
Related Questions
Q66: Determine Q67: The symbol Q68: Hydrochloric acid (HCl) reacts with sodium Q69: Processes are always spontaneous when _ Q70: Hydrochloric acid (HCl) reacts with sodium Q72: Determine the value of Q73: Determine the value of Q74: What is the maximum amount of work Q75: The reaction Q76: A reaction is at equilibrium at![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents