For the following exothermic reaction, predict under which conditions the reaction will be spontaneous. 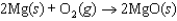
A) The reaction is always spontaneous.
B) The reaction is never spontaneous.
C) The reaction is spontaneous at high temperatures.
D) The reaction is spontaneous at low temperatures.
E) Insufficient data is provided to answer this question.
Correct Answer:
Verified
Q80: At constant T and P, any
Q81: One of the following statements A-D
Q82: At body temperature, many proteins have
Q83: For a chemical reaction with a positive
Q84: Alcohols for use as biofuels can be
Q86: Dinitrogen tetroxide (N2O4) decomposes to nitrogen
Q87: Alcohols for use as biofuels can
Q88: Because the triple point of water
Q89: Which statement characterizes the following table? Temperature
Q90: A reaction with a low enthalpy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents