At what temperature does the Fe(s) 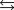 Fe(g) phase transition occur?
Fe(g) phase transition occur?
H = 415.5 kJ/mol; S = 153.4 J/mol · K.
A) 2,162 F
B) 2,435 F
C) 4,352 F
D) 4,416 F
E) 2,709 F
Correct Answer:
Verified
Q91: Which statement characterizes the following table? Temperature
Q92: A reaction is not spontaneous at
Q93: The entropy of vaporization of water is
Q94: The enthalpy and entropy of vaporization
Q95: Calcium sulfate is a desiccant used
Q97: When a solution of DNA in
Q98: For the following endothermic reaction, predict under
Q99: The dissolution of ammonium nitrate in water
Q100: A reaction with a low enthalpy
Q101: If
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents