Using the thermodynamic data below, determine the equilibrium constant for the conversion of hydrogen and oxygen to water at 500 K.
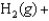
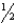

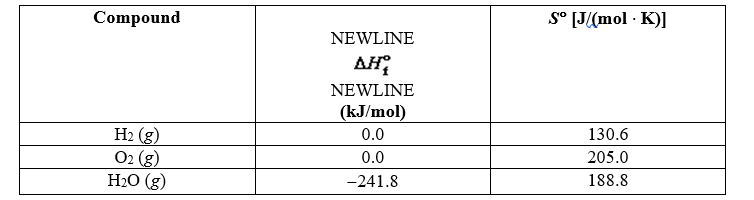
A) 1.13 10-23
B) 8.86 1022
C) 4.67 10-8
D) 3.25 1021
E) 3.08 10-22
Correct Answer:
Verified
Q105: For a particular hypothetical reaction,
Q106: A perturbation or stress to a chemical
Q107: If
Q108: Some pure metals can be obtained from
Q109: Suppose
Q111: If for a given chemical reaction
Q112: Using the thermodynamic data below, determine
Q113: Suppose
Q114: For a particular hypothetical reaction,
Q115: Which of the following must be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents