The values of 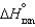 and
and 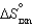 are shown for the following equilibrium reaction. What makes the equilibrium constant larger at low temperature?
are shown for the following equilibrium reaction. What makes the equilibrium constant larger at low temperature? 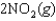
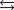
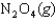 H = -58.02 kJ/mol, S = -176.5 J/mol . K
H = -58.02 kJ/mol, S = -176.5 J/mol . K
A) The negative value of H is dominant at all temperatures.
B) The negative value of S is dominant at all temperatures.
C) The negative value of S is combined with the small value of T.
D) The negative value of H is combined with the small value of T.
Correct Answer:
Verified
Q120: Benzene is a liquid under standard conditions.
Q121: If K for a reaction is
Q122: As the temperature of a reaction
Q123: A sketch of the free energy for
Q124: Jane can accept that
Q126: The equilibrium constant for a reaction
Q127: A qualitative interpretation of the effect
Q128: A sketch of the free energy
Q129: What is the value of the
Q130: As T approaches infinity, ln K approaches
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents