Calculate the equilibrium constant at 500 K for the following reaction given the data below. 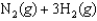
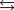
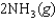 K c (298 K) = 6.1 105
K c (298 K) = 6.1 105 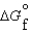 (NH3, g) = -16.5 kJ/mol
(NH3, g) = -16.5 kJ/mol 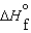 (NH3, g) = -46.1 kJ/mol
(NH3, g) = -46.1 kJ/mol
A) 53
B) 1,800
C) 7.5
D) 5.4 10-4
E) 332
Correct Answer:
Verified
Q134: As the temperature of an endothermic
Q135: When plotting ln K vs. 1/T,
Q136: Given the following two measurements of
Q137: What is the value of the
Q138: Which of the following relationships are
Q140: A sealed tube containing an equilibrium
Q141: What types of motion does a molecule
Q142: The entropy of fusion for ice
Q143: When you increase the volume of a
Q144: What type of motion is depicted in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents