Which statement about the reaction below, where en = ethylenediamine (H2NCH2CH2NH2) , is not correct? 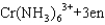
A) The reaction is product-favored because the entropy of the system increases.
B) The Lewis basicity of the amino groups in en is comparable to that of the ammonia molecules.
C) The reaction is reactant-favored because the concentration of ammonia on the right side is higher than the concentration of en on the left side.
D) The reaction enthalpy is small because the Cr-NH3 and Cr-en bond strengths are very similar.
E) Both Cr(NH3) 63+ and Cr(en) 33+have an octahedral coordination geometry and a coordination number of 6.
Correct Answer:
Verified
Q155: Which of the following states of motion
Q156: In a biochemical reaction, A +
Q157: Which of the following must be true
Q158: Give an example of a spontaneous and
Q159: Which of the following is the best
Q161: Hydrogen iodide can theoretically be made
Q162: Using the thermodynamic data below, determine
Q163: Carbon atoms can be found in a
Q164: Determine the change in the standard entropy
Q165: What are the signs of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents