What is indicated by the shape of the titration curve? 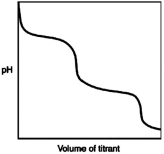
A) A diprotic acid was titrated with a strong base.
B) A triprotic acid was titrated with a strong base.
C) A diprotic base was titrated with a strong acid.
D) A triprotic base was titrated with a strong acid.
E) A strong acid was titrated with a strong base.
Correct Answer:
Verified
Q31: The following titration curve is most likely
Q32: Halfway to the equivalence point in a
Q33: A 25.0 mL solution of quinine was
Q34: The following titration curve is most likely
Q35: A 0.500 g sample of an unknown
Q37: In a titration of monoprotic acids and
Q38: When an acetic acid solution is titrated
Q39: What are the characteristics of a pH
Q40: You have a summer job as an
Q41: A solution of ammonia (0.12 M,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents