At what point in the following titration curve for a weak acid being titrated with a strong base is the pH equal to the pKa of the acid? The x-axis scale goes from 0.0 mL to 20.0 mL. The sharp rise is at 10.0 mL. 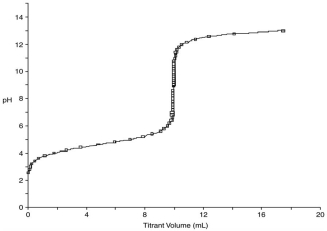
A) 0.0 mL
B) 5.0 mL
C) 9.0 mL
D) 10.0 mL
E) 18.0 mL
Correct Answer:
Verified
Q21: Acid-base indicators change color _
A)exactly when pH
Q22: Acid-base indicators need to have very intense
Q23: A solution of benzoic acid (0.21
Q24: The most suitable acid-base indicator for a
Q25: To simulate the pH of blood,
Q27: A solution of formic acid (0.15
Q28: Bromocresol green is yellow in its acidic
Q29: Glycolic acid, which is a monoprotic acid
Q30: Lactic acid, which is found in milk
Q31: The following titration curve is most likely
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents