Identify the Lewis acid in the following reaction: 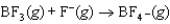
A) BF3
B) F-
C) BF4-
D) None of these is an acid.
E) All of these are acids.
Correct Answer:
Verified
Q38: When an acetic acid solution is titrated
Q39: What are the characteristics of a pH
Q40: You have a summer job as an
Q41: A solution of ammonia (0.12 M,
Q42: Identify the Lewis base in the following
Q44: A solution of sulfuric acid (H2SO4, 25.00
Q45: A phosphate buffer solution (25.00 mL sample)
Q46: One brand of extra-strength antacid tablets contains
Q47: A solution of hydrofluoric acid (0.27
Q48: A Lewis acid is _
A)a proton donor.
B)a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents