The acid ionization equilibrium constant, Ka, describes the reaction (where HA is a generic weak acid) ________
A) HA +OH-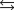 H2O +A-.
H2O +A-.
B) HA + H2O 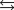 H3O+ + A-.
H3O+ + A-.
C) HA + H3O+ 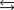 H2A- + H2O.
H2A- + H2O.
D) HA + H2A+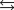 H2A-+ HA.
H2A-+ HA.
E) H3O+ +A-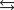 HA + H2O.
HA + H2O.
Correct Answer:
Verified
Q4: Which sketch best represents the qualitative molecular
Q5: In the following reaction in aqueous solution,
Q6: Which of the following is a strong
Q7: Which of the following is the conjugate
Q8: Ammonia (NH3) acts as a weak base
Q10: Which one of the following is a
Q11: Which sketch best represents the qualitative molecular
Q12: Three acids found in foods are lactic
Q13: Which of the following is the conjugate
Q14: Which one of the following is not
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents