Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 + H2O  H2PO4-+H3O+
H2PO4-+H3O+ 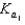 H2PO4- + H2O
H2PO4- + H2O  HPO42- + H3O+
HPO42- + H3O+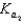 HPO42- + H2O
HPO42- + H2O  PO43-+ H3O+
PO43-+ H3O+
What is the Kb expression for the base, sodium phosphate?
A) 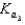
B) 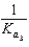
C) 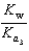
D) 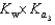
E) 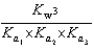
Correct Answer:
Verified
Q100: What is the Ka for the
Q101: Write the reaction and equilibrium constant that
Q102: What is the pH of a
Q103: What is the pH of a solution
Q104: Phosphoric acid is a triprotic acid, ionizing
Q106: Lactic acid is a major component of
Q107: What is the pH of a
Q108: What is the pH of a
Q109: What is the concentration of the
Q110: Effects that stabilize the conjugate base of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents