A chemical equilibrium 2 A 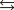 B
B
Has a forward rate constant, kf = 10 M -1s-1, and a reverse rate constant, kr = 5.0 s-1. What is the value of the equilibrium constant for this system?
A) 0.5
B) 2.0
C) 20
D) 0.050
E) 5.0
Correct Answer:
Verified
Q1: The forward rate constant, kf, and reverse
Q3: For an equilibrium reaction with K
Q4: A chemical equilibrium 2 A 
Q5: A chemical equilibrium may be established by
Q6: Which expression corresponds to the equilibrium constant
Q7: Which statement about a chemical reaction is
Q8: In a reversible reaction A
Q9: Write the equilibrium expression for the reaction
Q10: A chemical equilibrium A
Q11: The law of mass action is a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents