The equilibrium constant for the reaction below is 17.5.
S(s) + 3 O2(g) 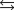 2 SO3(g)
2 SO3(g)
What is the value of the equilibrium constant for the following reaction?
6 SO3(g) 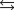 3S(s) + 9 O2(g)
3S(s) + 9 O2(g)
A) 1.87 10-4
B) 5.71 10-2
C) 17.5
D) 306
E) 5360
Correct Answer:
Verified
Q51: If the reaction quotient Q is equal
Q52: The equilibrium constant for the reaction
Q53: A series of four equilibrium steps
Q54: In equilibrium expressions, the concentrations of pure
Q55: Which one of the following is the
Q57: In the following reaction, which of
Q58: Gaseous hydrogen and iodine can be
Q59: Water can decompose at an elevated
Q60: If the reaction quotient Q has a
Q61: Which of the following reactions will not
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents