Given the following reactions and associated equilibrium constants 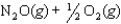
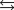 2NO(g) K1 = 1.70 10-13
2NO(g) K1 = 1.70 10-13 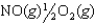
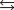 NO2(g) K2 = 6.83 106
NO2(g) K2 = 6.83 106
What is the equilibrium constant for the following reaction?
2N2O(g) + 3O2(g) 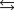 4NO2(g) Knew = ?
4NO2(g) Knew = ?
A) 1.59 10-2
B) 9.29 10-18
C) 40.3
D) 62.9
E) 1.08 1017
Correct Answer:
Verified
Q42: Given the following reactions and associated
Q43: If the reaction quotient Q has a
Q44: The equilibrium constants for the two
Q45: The chemical equilibrium constant for the
Q46: Which of the following is the equilibrium
Q48: Sulfur dioxide emitted in the burning of
Q49: Write the expression for the reaction quotient
Q50: Which statement about an equilibrium constant, K,
Q51: If the reaction quotient Q is equal
Q52: The equilibrium constant for the reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents