How would an increase in temperature affect the following equilibrium?
N2(g) + 3H2(g) 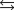 2NH3(g)
2NH3(g) 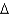 H° = -92.0 kJ/mol
H° = -92.0 kJ/mol
A) More product would form and K would increase.
B) More product would form but K would remain constant.
C) K would be unaffected by a temperature change.
D) More reactants would form but K would remain constant.
E) More reactants would form and K would decrease.
Correct Answer:
Verified
Q74: Addition of reactants to a chemical reaction
Q75: Identify whether or not perturbations A-D will
Q76: Increasing the temperature of an exothermic reaction
Q77: Which of the following occurs when reactants
Q78: Addition of products to a chemical reaction
Q80: How would an increase in temperature
Q81: A reaction is run under conditions
Q82: The sulfide ion, S2-, reacts with water
Q83: To solve an equation of the form
Q84: When can an x be ignored in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents