In the Reaction, Initial, Change, Equilibrium (RICE) table started for calculating equilibrium concentrations of the reaction shown, the terms in the "change" row are ________ 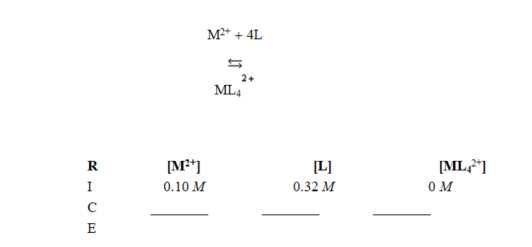
A) (-x, -x, +x.)
B) (+x, +x, -x.)
C) (-x, - 4x, +x.)
D) (+x, +4x, +x.)
E) (+x, +4x, -x.)
Correct Answer:
Verified
Q65: If the temperature of an endothermic reaction
Q66: Given the reaction below, which of the
Q67: Given the reaction below, which of the
Q68: Carbon dioxide and argon, which is a
Q69: What happens to the equilibrium between NO2(g)
Q71: Solid mercury(II) oxide decomposes when heated to
Q72: Which of the following is true regarding
Q73: For a chemical reaction at equilibrium, which
Q74: Addition of reactants to a chemical reaction
Q75: Identify whether or not perturbations A-D will
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents