Given the reaction below, explain how the equilibrium would be reestablished after each of the following stresses are applied.
4NH3(g) + 3O2(g) 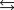 2N2(g) + 6H2O(g)
2N2(g) + 6H2O(g) 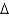 H° = -12.0 kJ/mol
H° = -12.0 kJ/mol
a) Addition of water
b) Removal of ammonia
c) Increase of the volume of the container
d) Lowering of the reaction temperature
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q100: The reaction of bromine gas with chlorine
Q101: If Q for a reaction is greater
Q102: Hydrofluoric acid, HF, dissociates in water
Q103: Students often write expressions for the equilibrium
Q104: Consider the reaction of nitrogen and hydrogen
Q106: The sulfide ion, S2- reacts with
Q107: In the following reaction, calculate Q, given
Q108: How and why are the expressions for
Q109: The reaction of bromine gas with chlorine
Q110: Which of the listed perturbations will change
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents