One reaction that occurs in an automobile catalytic converter is the conversion of carbon monoxide to carbon dioxide. How is the rate of this reaction related to the rate at which the concentration of a reactant or product changes?
2CO(g) + O2(g) →2CO2(g)
I. Rate =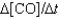
II. Rate = 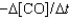
III. Rate = 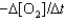
IV. Rate =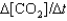
A) I only
B) II only
C) III only
D) II and III only
E) II, III, and IV only
Correct Answer:
Verified
Q3: Indicate which of the following compounds is
Q4: Which of the following is not important
Q5: For the reaction 2A + 3B
Q6: NO2 contributes to the "brown haze" associated
Q7: For the reaction 2A 
Q9: Large cities often issue ozone advisories. At
Q10: NO2 concentrations during photochemical smog events often
Q11: Octane can undergo combustion to produce carbon
Q12: The greatest NO concentration is observed _
A)in
Q13: At what time of day are ozone
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents