If the rate of formation of ammonia is 0.345 M/s, what is the rate of disappearance of N2? 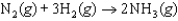
A) 0.173 M/s
B) 0.345 M/s
C) 0.690 M/s
D) 245 M/s
E) 0.518 M/s
Correct Answer:
Verified
Q35: A scientist conducts an experiment to determine
Q36: If the rate of formation of dinitrogen
Q37: If the rate of formation of nitrogen
Q38: If the rate of formation of ammonia
Q39: Using the reaction shown below, which statement
Q41: The rate law of a particular reaction
Q42: For the rate law Rate = k[A][B]1/2,
Q43: The half-life (t1/2) of a first-order reaction
Q44: In a rate law, the partial orders
Q45: If the reaction 3A + B +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents