If the reaction 3A + B + 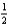 C
C  2D + E is first order overall, which of these could be the units of its rate constant, k?
2D + E is first order overall, which of these could be the units of its rate constant, k?
A) 1/s
B) M/s
C) 1/Ms
D) 1/Ms2
E) Ms
Correct Answer:
Verified
Q40: If the rate of formation of ammonia
Q41: The rate law of a particular reaction
Q42: For the rate law Rate = k[A][B]1/2,
Q43: The half-life (t1/2) of a first-order reaction
Q44: In a rate law, the partial orders
Q46: In a first order reaction, the
Q47: A second-order reaction (2A Q48: The reaction A(g) + B(g) Q49: If the rate of formation of ammonia Q50: The reaction ![]()

![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents