Which of the following plots would indicate that a reaction was first order?
A) 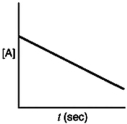
B) 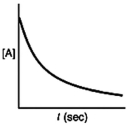
C) 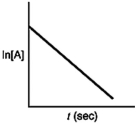
D) 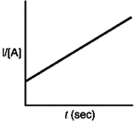
Correct Answer:
Verified
Q57: The rate law of a particular reaction
Q58: The reaction CHCl3(g) + Cl2(g) 
Q59: The disappearance of HI in the reaction
Q60: Which of these could be the units
Q61: Which of the following plots indicates that
Q63: The linear form of the Arrhenius equation
Q64: Which of the following plots indicates that
Q65: Chlorine dioxide (ClO2) is used as a
Q66: Which plot would provide the activation energy
Q67: N2O5 is used as a source
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents