Which point in the reaction profile below represents an intermediate? 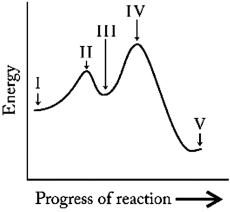
A) II and IV
B) II only
C) III
D) IV only
E) V
Correct Answer:
Verified
Q69: How many steps are involved in the
Q70: Which point as labeled by an asterisk
Q71: The energy profiles for four different reactions
Q72: The energy profiles for four different reactions
Q73: Collision theory assumes that the rate of
Q75: The energy needed to form an activated
Q76: Sodium hypochlorite (NaClO) is used as a
Q77: Consider the rate law expression Rate =
Q78: The first-order reaction A Q79: The linear form of _ is very![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents