The following figure shows Arrhenius plots for four different reactions. Which reaction has the lowest activation energy? 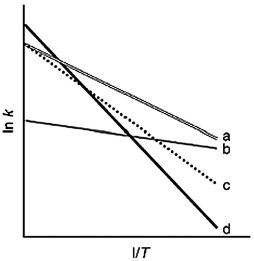
A) a
B) b
C) c
D) d
Correct Answer:
Verified
Q95: The following figure shows Arrhenius plots for
Q96: The energy profiles for four different reactions
Q97: The following figure shows Arrhenius plots for
Q98: The following figure shows Arrhenius plots for
Q99: The reaction profiles for four reactions are
Q101: Given the following data for the reaction
Q102: The mechanism for the reaction 
Q103: What is the predicted rate law for
Q104: The reaction Q105: The mechanism for the reaction ![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents