The reaction 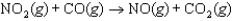
is thought to occur by the following mechanism:
Step 1: 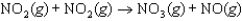
Step 2: 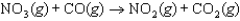
Which of the following species is an intermediate?
A) NO2
B) NO
C) NO3
D) CO2
E) This mechanism has no intermediates.
Correct Answer:
Verified
Q99: The reaction profiles for four reactions are
Q100: The following figure shows Arrhenius plots for
Q101: Given the following data for the reaction
Q102: The mechanism for the reaction 
Q103: What is the predicted rate law for
Q105: The mechanism for the reaction 
Q106: A proposed mechanism for the decomposition of
Q107: A reaction mechanism consists of a series
Q108: Given the following data for the reaction
Q109: Given the following data for the reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents