A solution is prepared by mixing 75 g of benzene (C6H6) with 25 g of toluene (C7H8) . Use the following data to determine the vapor pressure of this solution at 20 C. 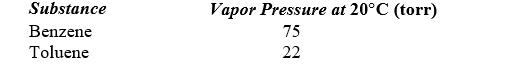
A) 63 torr
B) 49 torr
C) 87 torr
D) 35 torr
E) 71 torr
Correct Answer:
Verified
Q70: The vapor pressure of an aqueous
Q71: Which of the following is a colligative
Q72: A colligative property does not depend on
Q73: Calculate the molality of a solution containing
Q74: Which statement regarding the fractional distillation of
Q76: Isopropyl alcohol has a boiling point
Q77: Indicate which aqueous solution has the slowest
Q78: A petroleum company separates benzene (78
Q79: Calculate the molality of a solution
Q80: A solution is prepared by mixing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents