Which of the following diagrams best shows a set of polar molecules interacting through dipole-dipole interactions?
A) 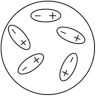
B) 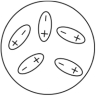
C) 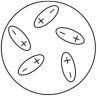
D) 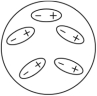
Correct Answer:
Verified
Q11: Which molecule does not exhibit hydrogen bonding?
A)HF
B)CH3NH2
C)CH3OH
D)(CH3)3N
E)CH3CH2OH
Q12: Which of the following compounds is not
Q13: When potassium bromide dissolves in water, which
Q14: A hydration sphere forms around an ion
Q15: Which of the following compounds is capable
Q17: Which molecule has the largest dipole?
A)methane (CH4)
B)ammonia
Q18: Dipole-dipole interactions typically are not as strong
Q19: Which of the following compounds is capable
Q20: Which of the following solvents will involve
Q21: Polarizability refers to _
A)the ease with which
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents