If water contains about 42 mg of oxygen per liter at 20 C and 1.0 atm, what would be the value of Henry's law constant for oxygen dissolving in water? The mole fraction of oxygen in air is 0.21.
A) 0.25 mol/(L atm)
B) 1.3 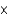 103 mol/(L atm)
103 mol/(L atm)
C) 1.9 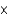 10-2 mol/(L atm)
10-2 mol/(L atm)
D) 6.3 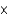 10-3 mol/(L atm)
10-3 mol/(L atm)
E) 0.010 mol/(L atm)
Correct Answer:
Verified
Q37: When two liquids mix completely in all
Q38: Which best describes the intermolecular forces present
Q39: Of all the noble gases, _ has
Q40: Molecular nitrogen (N2) interacts with water and
Q41: The solubility of any gas in a
Q43: Which of the following gases do you
Q44: Which of the following compounds would you
Q45: The concept "like dissolves like" explains why
A)I2
Q46: Which of the following compounds would be
Q47: Of the two compounds shown below, one
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents