The Henry's law constant (mol/L · atm) for carbon dioxide dissolving in water is 3.50 10-2 mol/(L atm) at 20 C. Calculate the molar concentration of carbon dioxide in a soda can where the air pressure is 2.45 atm. The mole fraction of carbon dioxide in air is 0.0397.
A) 1.4 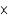 10-3 M
10-3 M
B) 3.4 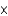 10-3 M
10-3 M
C) 2.7 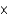 10-2 M
10-2 M
D) 8.2 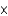 10-2 M
10-2 M
E) 8.6 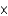 10-2 M
10-2 M
Correct Answer:
Verified
Q54: Which of the following gases do you
Q55: Henry's law constant (mol/L · atm)
Q56: Which of the following pairs of compounds
Q57: The pressure inside a bottle of carbonated
Q58: Air (consisting mostly of nitrogen (N2), oxygen
Q60: Indicate which of the following pairs of
Q61: Which alkane compound has the highest boiling
Q62: Which plot shown below would be useful
Q63: The smell of fresh-cut pine is due
Q64: Gasoline is primarily a mixture of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents