The carbon dioxide pressure in a bottle of champagne is about 6 atm. At this pressure about 0.45 g of carbon dioxide dissolves in 100 mL of champagne. How much carbon dioxide remains dissolved in 100 mL after the bottle is opened? The partial pressure of carbon dioxide then is 6.0 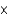 10-4 atm.
10-4 atm.
A) 0.45 g
B) 3.8 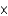 10-3 g
10-3 g
C) 1.7 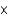 10-6 g
10-6 g
D) 9.0 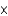 10-4 g
10-4 g
E) 4.5 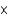 10-5 g
10-5 g
Correct Answer:
Verified
Q46: Which of the following compounds would be
Q47: Of the two compounds shown below, one
Q48: Which of the following compounds do you
Q49: Which of the following compounds would be
Q50: Which of the following statements does not
Q52: Which alcohol should be most soluble in
Q53: The pressure of carbon dioxide in a
Q54: Which of the following gases do you
Q55: Henry's law constant (mol/L · atm)
Q56: Which of the following pairs of compounds
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents