The phase diagram for carbon dioxide is shown below. What are the phase changes in order as carbon dioxide is heated from -90oC to 50oC, at 500 atm pressure? 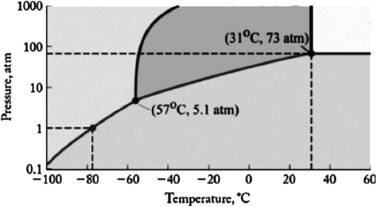
A) solid  liquid
liquid  gas
gas  supercritical fluid
supercritical fluid
B) solid  liquid
liquid  supercritical fluid
supercritical fluid
C) solid  gas
gas
D) gas  liquid
liquid  solid
solid
E) liquid  gas
gas
Correct Answer:
Verified
Q96: The temperature at point a in the
Q97: On the phase diagram below, identify the
Q98: Sublimation occurs in going from region _
Q99: Which of the following substances has a
Q100: Point c in the phase diagram below
Q102: For molecules or atoms with the same
Q103: Carbon dioxide is being used as an
Q104: Which is the dominant interaction that explains
Q105: The phase diagram for carbon dioxide is
Q106: The density of water decreases as
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents