The phase diagram for carbon dioxide is shown below. What are the phase changes in order as the pressure is increased starting at 0.5 atm, and the temperature is kept at -80oC? 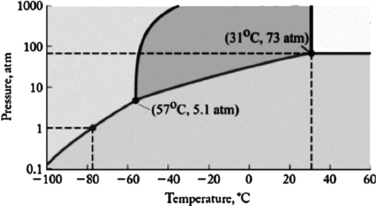
A) solid  gas
gas
B) solid  liquid
liquid  gas
gas
C) liquid  gas
gas
D) gas  solid
solid
E) gas  liquid
liquid
Correct Answer:
Verified
Q108: Which is the dominant interaction between water
Q109: Viscosity is a measure of a substance's
Q110: The resistance of a liquid to an
Q111: Which type of intermolecular interaction exists for
Q112: Which is the dominant interaction that leads
Q114: Which is the dominant interaction between chloroform
Q115: The phase diagram for carbon dioxide is
Q116: Which is the dominant interaction between oxygen
Q117: For molecules, atoms, or ions with the
Q118: Water forms a concave meniscus in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents