What is the formal charge of each atom (from left to right) in the following resonance structure of SN2? 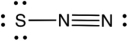
A) 0, 0, 0
B) (-1, +1, 0)
C) (-2, +1, 0)
D) (-2, -1, +1)
E) (-1, -1, +1)
Correct Answer:
Verified
Q82: Which of the following pairs of Lewis
Q83: Which structure for dinitrogen sulfide (N-N-S or
Q84: What is the formal charge on the
Q85: What is the formal charge of each
Q86: Nitrite (NO2-) is an important nutrient in
Q88: Which statement about the most stable Lewis
Q89: O-S-S-O has three resonance Lewis structures that
Q90: What is the formal charge of the
Q91: Use the formal charge as a criterion
Q92: One of the resonance structures of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents