Based on consideration of formal charges, which of the following is the most stable Lewis structure for the azide ion (N3-) ?
A) 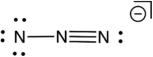
B) 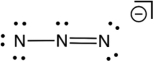
C) 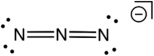
D) 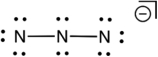
Correct Answer:
Verified
Q100: What is the formal charge of the
Q101: Which of the following, based on formal
Q102: How many lone-pair electrons are on the
Q103: Which of the following is the shortest
Q104: In which one of the following molecules
Q106: Which of the following represents the best
Q107: Which one of the molecules below has
Q108: Which one of the following molecules violates
Q109: In comparing the length and strength of
Q110: Which one of the following molecules violates
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents