The 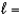 3 subshell contains how many total electrons?
3 subshell contains how many total electrons?
A) 2
B) 6
C) 10
D) 14
E) 18
Correct Answer:
Verified
Q65: Subshells are _
A)orbitals with the same principal
Q66: De Broglie reasoned that for the electron
Q67: An orbital's shape is determined by _
A)the
Q68: Which statement about the quantum numbers that
Q69: How many orbitals are possible for the
Q71: A proton in a cyclotron has a
Q72: Which subshell only has five orbitals?
A)
Q73: A shell consists of all _
A)electrons with
Q74: How many orbitals are possible for the
Q75: Which subshell contains six total electrons?
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents