Which combination of quantum numbers is possible for an atom with five orbitals in one subshell?
A) n  1,
1, 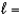 0
0
B) n  2,
2, 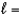 4
4
C) n  3,
3, 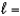 2
2
D) n  4,
4, 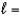 4
4
E) n  5,
5, 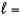 0
0
Correct Answer:
Verified
Q94: How many orbitals that have the principal
Q95: Which of the following is not a
Q96: Which of the following is not an
Q97: A certain shell is known to have
Q98: What ml quantum numbers are possible when
Q100: What is the orbital designation for an
Q101: Which of the following orbitals is the
Q102: Which orbital has the lowest energy in
Q103: What is the ground state electron configuration
Q104: Which one of the following diagrams represents
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents