Which of the following is a possible set of quantum numbers for a 3d orbital?
A) n  3,
3, 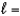 1, me
1, me
 1
1
B) n  3,
3, 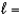 0, me
0, me  0
0
C) n  3,
3, 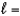 2, me
2, me  3
3
D) n  3,
3, 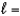 2, me
2, me  0
0
E) n  3,
3, 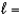 1, me
1, me  2
2
Correct Answer:
Verified
Q87: Which of the following represents a p
Q88: What Q89: A certain shell is known to have Q90: How many orbitals exist for the quantum Q91: Which of the following is not a Q93: If the principal quantum number is seven Q94: How many orbitals that have the principal Q95: Which of the following is not a Q96: Which of the following is not an Q97: A certain shell is known to have![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents